Most people have bruised their skin and as a result gotten a lifelong scar that is not so pleasant in appearance. Scarring is the end result of a wound healing process in human adults. Research show that scars are largely due to a protein family group of extracellular matrix proteins, the most known protein of this family is collagen (25-35%). Dysfunctional extracellular matrix protein composition during wound healing, leads to loss of function of the tissue and to a drastic change in cosmetic appearance. Scarring or the more general term – fibrosis is the end result of dysfunctional healing at the protein level.
Understanding the complex mechanism of wound healing or scar-reversing is not of primary clinical importance in skin disease but in other fibrotic diseases. For example, cirrhosis is a severe fibrosis seen in final stage of chronic liver disease. It is an increasing cause of morbidity and death worldwide, currently the 14th most common cause of death. In depth study of fibrosis biology is of importance to find novel methods to reverse these diseases.
Dr. Larsson, with colleagues, show in a recent manuscript a label-free cathodoluminescence method, which is capable to visualize extracellular matrix proteins, and help better understanding of wound healing and other pathological diseases (Zielinski et al. 2019).
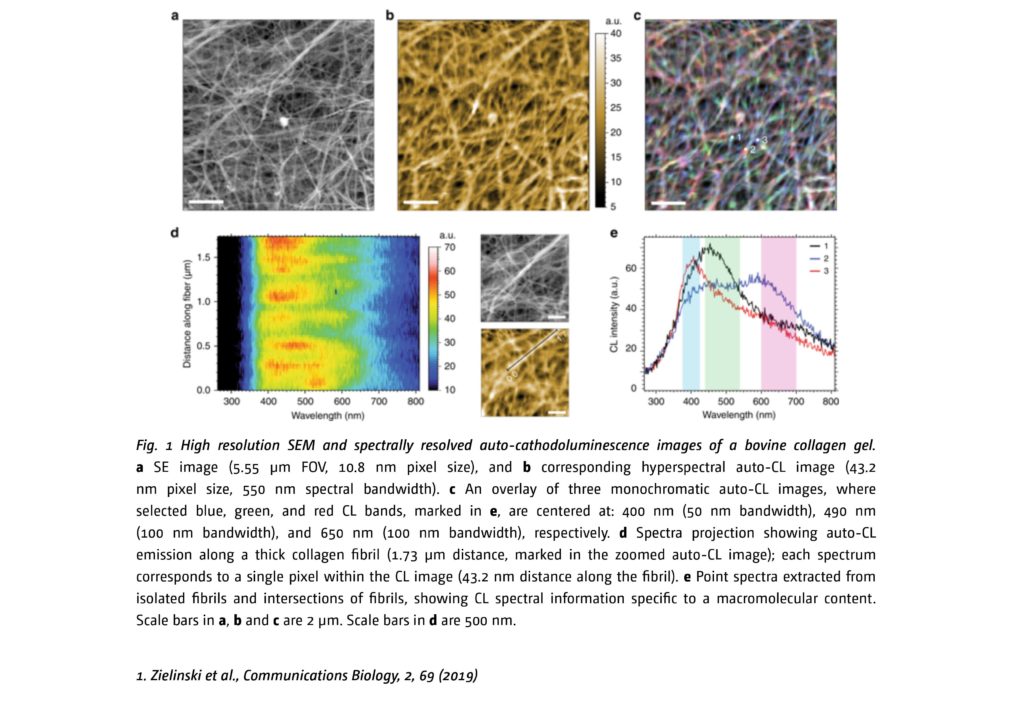
The manuscript describes the use of cathodoluminescence (CL) – a spectroscopic effect occurring in a scanning electron microscope (SEM), applied with a label-free approach in detection of light emitted by collagen tissue samples. Spectrally analyzed color distribution of the intrinsic light emission from isolated fibrils at a very tight spot of few nm, unveils unique macromolecular biological components in a highly heterogenous extracellular matrix, as showed in the figure 1. This concept is called auto-cathodoluminescence, which can be described as an analogue of auto-fluorescence emission, a well-known effect in the field of optical microscopy. “In layman terms” – says Dr. Larsson – “with auto-cathodoluminescence we cannot only see, but also identify and localize specific types of macromolecules in highly diverse biological samples, such as a tissue biopsy.”
The idea of utilizing cathodoluminescence in characterization of bio-organic materials is a re-discovered concept, initially proposed by Pease and Hayes in 1966. The reports from that time showed CL spectra of few types of amino acids, nucleotides and DNA, yet until now, CL bioimaging capabilities were always drastically limited due to the available technology. The high-end Attolight CL technology has proved to deliver the missing chapter to this “old” bio-CL story. The main finding is that bio-CL imaging can reach the spatial resolution of that of the STED or a similar super resolution optical method, without a need for staining.
This gives a clear advantage, since contrast-enhancing molecules, e.g. fluorescing antibodies, can spot only a specific type of a specimen for which they have been designed. Another drawback of nanolabels, is that in order to provide enough brightness, their size often goes beyond the size of a macromolecule of interest.
In a modern biology research, we look at complex networks of interacting molecules, but we are still lacking tools to capture and fully understand these molecular complexities.
Label-free cathodoluminescence technology opens a new alley for analytical methods, and it appears to be capable of identifying macromolecules, and spotting them with a spatial resolution never achieved by any of the spectroscopic methods applied in life sciences. Continuation of this first study on label-free cathodoluminescence will aim at establishing novel analytical methodology, especially in supporting drug development processes and improve our understanding of fibrosis.
Open-access publication available at: https://www.nature.com/articles/s42003-019-0313-x